Abstract
We report the first prospective, multi-center, single-arm phase I/II study that assesses the safety and feasibility of haploidentical transplantation with TCRalpha/beta and CD19+ cells depleted peripheral blood stem cell grafts using the CliniMACS plus System (Miltenyi Biotec, Germany) in combination with a reduced-intensity conditioning (RIC) (www.clinicaltrialsregister.org; 2011-005562-38). The grafts were processed in 7 GMP laboratories and immune cell assays were performed in two core labs using standardized methods and the MACSQuant flow cytometry device (Miltenyi Biotec, Germany). The RIC regimen consisted of 15 or 30 mg ATG (Fresenius/Grafalon) or 7 Gy total nodal irradiation, 160 mg/m2 fludarabine, 10 mg/kg thiotepa, and 140 mg/m2 melphalan. MMF (40mg/kg/day) was used for GVHD prophylaxis until Day 30. The Day 100 data of the 30 pediatric patients have been presented at ASH2016; this report presents the data of the 30 adult patients with at least 100 days follow-up alive.
Results: Seven hospitals enrolled 13 female and 17 male participants (median age 38.5 years, range 20-63 years), including 7 ALL, 17 AML, 3 MDS/MPS, 1 each with multiple myeloma, acute undifferentiated leukemia or sickle cell anemia. Malignant diseases were in complete remission (n=19), partial remission (n=6) or non-remission (n=4). Eight had one or more previous SCTs. The median follow-up is 360 days (range, 18 - 776). Of the 30 recipients, 9 already completed the 2-year follow-up.
The median number of CD34+ cells, TCRab+ cells and CD20+ cells/kg was 8.1 x 106 (range, 4 - 18), 13.5 x 103 (range, 0.22 - 64.3) and 28 x 103 (range, 2 - 727), respectively. In addition, significant numbers of NK and TCRgd+ cells/kg were infused: median 39.3 x 106 (range, 9.4 - 102) and 8.9 x 106 (range, 1.1 - 30.7), respectively. All 30 patients tolerated the cell infusion well and 28 had primary engraftment of ANC >500 cells/µL at a median of 14 days (range, 9 - 41) and PLT >20,000 cells/µL at a median of 15 days (range, 12 - 38). Peripheral donor T-cell chimerism at the time of engraftment was complete in 27 patients, and mixed in one. On day 100, chimerism was completely donor in 24 patients and mixed in one. Two patients experienced primary graft failure after conditioning with ATG (one had sickle cell anemia, received 16 x 106 CD34 cells/kg; the other had high-risk AML in partial remission, received 18 x 106 CD34 cells). One patient had a secondary graft failure. All three patients were successfully re-transplanted.
No patient developed severe acute GVHD grade III/IV. Five patients (16.7%) had grade II, 7 (23.3%) had grade I, and 18 (60%) had none despite minimal GVHD prophylaxis after transplantation.
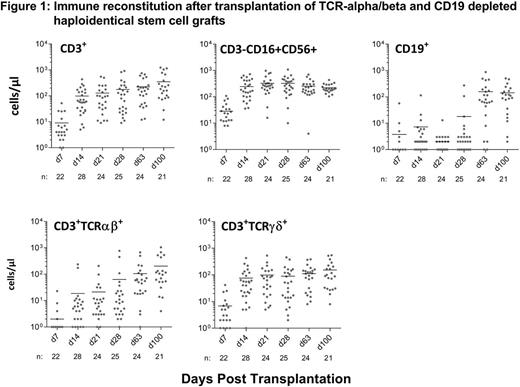
28 patients with primary engraftment were evaluable for immune reconstitution . The majority of lymphocytes on Day28 were NK cells (median 272 cells/µL; range, 10 - 1092); the median CD3+ cell count was 112 cells/µL (range, 9 - 911), with approximately two third of them being TCRgd+ (median 87 cells/µL; range, 7 - 891). Between day 28 and day 100, the median TCRab+ cells increased from 36 to 116 cells/µL (Figure 1).
CMV (n=13) and HHV6 (n=10) reactivations were the most common infectious complications following transplantation. However, only one patient developed CMV colitis. Similarly, although ADV was detected in 4 patients in stool, no ADV disease occurred. Two patients were tested positive for BKV and both experienced hemorrhagic cystitis. Two patients had EBV reactivation and 3 had bacterial sepsis.
At a median follow-up of 360 days 21 of 30 patients are alive; 9 patients died: 4 relapses, 5 organ failures (2 lung, 1 each liver, CNS, cardiac). After 100 days and 1 year the cumulative incidences of NRM and relapse were 7% and 8% and 20% and 13%, respectively.
Kaplan-Meier estimated OS and DFS were 93% and 86% on day 100 and 73% and 70% at 1 year, respectively.
Conclusion: The CliniMACSplus TCRalpha/beta and CD19-depletion System, when used in combination with a reduced-intensity conditioning regimen, resulted in favorable rates of primary engraftment (93%), no acute GVHD grade III/IV (0%), NRM (7%) and OS of 93% at day 100 and an estimated OS of 73% after 1 year. The immune reconstitution of innate immunity was rapid and there was no lethal viral and fungal infection. Longer follow up will provide more information on chronic GVHD and long term survival.
Bethge: Neovii GmbH: Honoraria, Research Funding; Miltenyi Biotech GmbH: Consultancy, Honoraria, Research Funding. Mielke: ISCT: Other: Travel support; DGHO: Other: Travel support; Cellex GmbH: Other: Travel grants, Speakers Bureau; Gilead: Other: Travel grants; MSD: Consultancy, Other: Travel grants; Celgene: Other: Travel grants, Speakers Bureau; Novartis: Consultancy; Jazz Pharma: Speakers Bureau; KIADIS Pharma: Other: Travel grants. Kuball: Gadeta (www.gadeta.nl): Consultancy, Equity Ownership, Patents & Royalties: on gdT cells and receptors and isolation strategies , Research Funding; Miltenyi: Research Funding. Karitzky: Miltenyi Biotec GmbH: Employment. Holtkamp: Miltenyi Biotec GmbH: Employment. Siewert: Miltenyi Biotec GmbH: Employment. Pflitsch: Miltenyi Biotec GmbH: Employment. Handgretinger: Miltenyibiotec GmbH: Patents & Royalties: Co-patent holder of TcR alpha/beta depletion technologies, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.